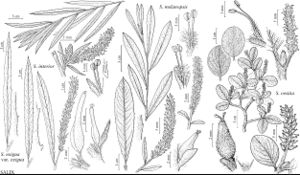
Salix interior
Bull. Torrey Bot. Club 27: 253. 1900.
Shrubs or trees, 4–9 m. Stems: branches gray-brown to redbrown, glabrous or villous; branchlets yellow-brown to redbrown, densely tomentose or villous to glabrescent. Leaves: stipules absent or rudimentary on early ones, rudimentary or foliaceous on late ones; petiole 1–5 (–9) mm, glabrous or sparsely villous adaxially; largest medial blade linear to lorate, 60–160 × 4–11 mm, (6.5–) 11–19 (–31) times as long as wide, base cuneate, margins flat, remotely spinulose-serrulate (teeth 2–5 per cm), apex acute or subacuminate, abaxial surface thinly glaucous, densely villous or long-silky to glabrescent, adaxial slightly glossy, pilose or densely villous to glabrescent; proximal blade margins entire; juvenile blade reddish or yellowish green, moderately densely to sparsely long-silky abaxially. Catkins (flowering throughout season); staminate 20–61 × 4–10 mm, flowering branchlet 3–20 mm; pistillate loosely flowered, slender or stout, 20–67 × 5–9 mm, flowering branchlet 3–19 mm; floral bract (sometimes greenish), 1.5–3.5 mm, apex acute, acuminate, or rounded, entire, erose, or toothed, abaxially hairy either proximally or distally, hairs wavy. Staminate flowers: abaxial nectary 0.5–1.1 mm, adaxial nectary ovate, narrowly oblong, or flask-shaped, 0.6–1.4 mm, nectaries distinct; filaments hairy; anthers 0.4–0.9 mm. Pistillate flowers: adaxial nectary narrowly oblong, 0.4–1.1 mm, shorter to longer than stipe; stipe 0.4–0.8 mm; ovary obclavate to pyriform, glabrous, glabrescent, or long-silky, beak abruptly tapering to styles; ovules 16–36 per ovary; styles 0–0.2 mm; stigmas flat, abaxially non-papillate with pointed tip, or broadly cylindrical, 0.3–0.7 mm. Capsules (4–) 5–8 (–10) mm. 2n = 38.
Phenology: Flowering early Apr-early Jul.
Habitat: Sandy to silty flood plains, margins of lakes, ponds, and prairie sloughs, dry prairie sand hills, marshes, disturbed areas
Elevation: 10-1800 m
Distribution
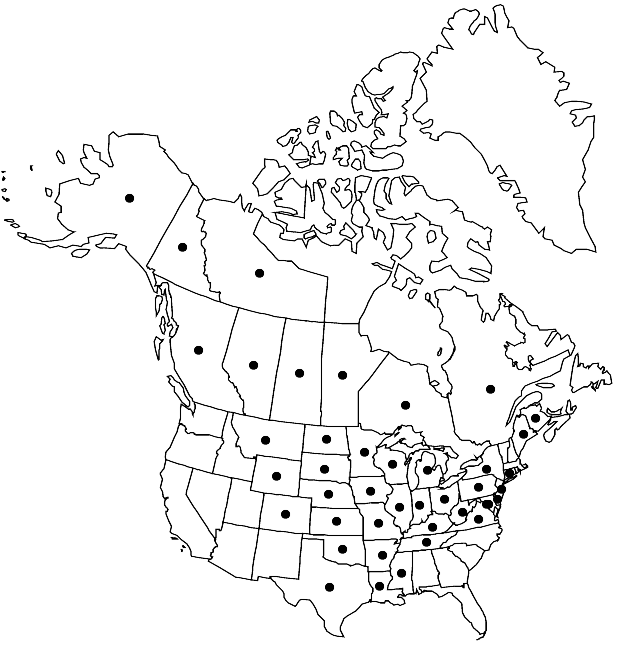
Alta., B.C., Man., N.B., N.W.T., Ont., Que., Sask., Yukon, Alaska, Ark., Colo., Conn., Del., D.C., Ill., Ind., Iowa, Kans., Ky., La., Maine, Md., Mich., Minn., Miss., Mo., Mont., Nebr., N.J., N.Y., N.Dak., Ohio, Okla., Pa., S.Dak., Tenn., Tex., Va., W.Va., Wis., Wyo., Mexico (Tamaulipas), Mexico (Veracruz)
Discussion
Sometimes Salix interior is treated as a subspecies of S. exigua (R. D. Dorn 1998). Salix exigua and S. interior hybridize and apparently intergrade in the western Great Plains; because the area of overlap is relatively small and distinctiveness of the two taxa is not compromised by hybridization and introgression, it is best to treat them as separate species.
Leaves on sylleptic shoots are usually very densely silky. Salix interior sometimes has shoots that arise from buds on either side of the normal axillary bud. They do not seem to be directly related to the stipules because they are enclosed by the petiole. Catkins with both staminate and pistillate flowers are rare in S. interior, but a Quebec specimen had some catkins predominantly pistillate and others staminate; most were a mixture. The flowers were not teratological, but a mature capsule contained aborted ovules.
Hybrids:
Salix interior forms natural hybrids with S. exigua var. exigua. Controlled pollinations using S. interior (as S. exigua) from southern Ontario (A. Mosseler 1990) successfully produced F1 hybrids with S. bebbiana, S. discolor, S. eriocephala, and S. petiolaris. Seed production was usually relatively low, except in crosses with S. discolor. In general, F1 viability was relatively low in crosses with these members of subg. Vetrix. No seeds were produced in crosses with members of subgenera Protitea or Salix. Morphology of the hybrids usually was intermediate between the two parents, but when S. petiolaris was used as the maternal parent, the F1s more closely resembled that species. J. Salick and E. Pfeffer (1999) extended these findings to show that, although crosses between S. interior (as S. exigua) and S. eriocephala are partially sterile, their clonal growth parameters (sprouting, shoot length, and biomass production) are strong and thus permit these partially sterile hybrids to exist as successful individuals and perhaps to “... make a contribution to interspecific gene flow over time.” Of particular taxonomic interest is that, in this cross, the staminate parent has a significant influence on leaf shape, whereas in the cross S. eriocephala × S. petiolaris it is the pistillate parent that is significant for leaf shape. Relatively few hybrids resembling those produced by Mosseler have been recognized in nature, but it is possible that the unusually broadly leaved plants named S. interior var. exterior and var. wheeleri, from northern Maine, Nebraska, New York, and West Virginia, and probably elsewhere, may be hybrids. Phenological isolation may be strong enough to prevent crosses in nature (A. Mosseler and C. S. Papadopol 1989) with the earlier flowering S. eriocephala and S. petiolaris, a barrier that even an occasional period of overlap cannot breach.
Selected References
None.